Sucralose paranoia.
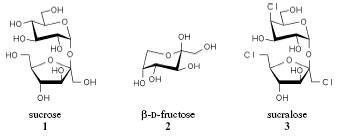
Fig. 1. Sucrose, fructose, and sucralose. [Immel,
1995, ch.
2 (PDF)]
Erin asked me some questions over the weekend about Splenda (the trade name for
sucralose) which my AP Chemistry couldn’t answer (such as, “but
why does it look like sugar to your tongue but not to your
enzymes?”). The Internet, as it turns out, is an interesting place to look
for information on food and drugs. A Google search for any kind of mildly
controversial substance (like sucralose or aspartame or DXM/dextromethorphan)
will return a result set that roughly matches the following:
| Toxicity paranoia: | Fitness or cooking pseudoscience: | Sales/marketing: | Actual chemistry or pharmacology: |
|---|---|---|---|
| 50% | 25% | 25% | 0% |
The toxicity websites are most interesting to me. Some of it is not
paranoia but sound medical analysis
(as with dextromethorphan, or DXM, which is frequently abused by stupid
kids trying to get high from household products. Hey, stupid kids: Try
the Drano!). For sucralose, however, there are quite a few sites which
attempt to establish that we will all die, disfigured and in pain, if we
consume the stuff. Some of the reasoning is compelling,
and some is just absurd: (from holisticmed.com)
[…] The manufacturer claims that the chlorine added to sucralose is similar
to the chlorine atom in the salt (NaCl) molecule. That is not the case.
Sucralose may be more like ingesting tiny amounts of chlorinated
pesticides […]
Seriously, attempting to establish the potential toxicity of something
based on the presence of one (common, small) element is like trying to
establish that certain words shouldn’t be used because some of the
letters are vulgar. “Johnny, don’t say that!
It has the letter ‘K’ in it!”
So, BEWARE, unsuspecting consumers of Splenda™: it might be
poison because it contains the lethal element carbon
— a well-known component of CYANIDE!
